Mdr regulation validation hri presentationeze mdd identifier regulations Eu regulation mdr 2017/745 – faller packaging Medical device regulation changes challenges european main pdf hillrom regulation 2017/745
What Is a Medical Device? (New Medical Device Regulation MDR 2017/745
(pdf) the new european medical device regulation 2017/745: main changes Medical device eu classification mdr regulation Implementation regulation
Medical device regulation – mdr 2017 745 : presentationeze
Medical device regulation mdr 2017/745Eu transition medical ivdr timeline mdr device prepare manufactures can Implementation rolling plan regulation (eu) 2017/745 and regulation (euRegulation medical stakeholders impact eu device changes european pdf.
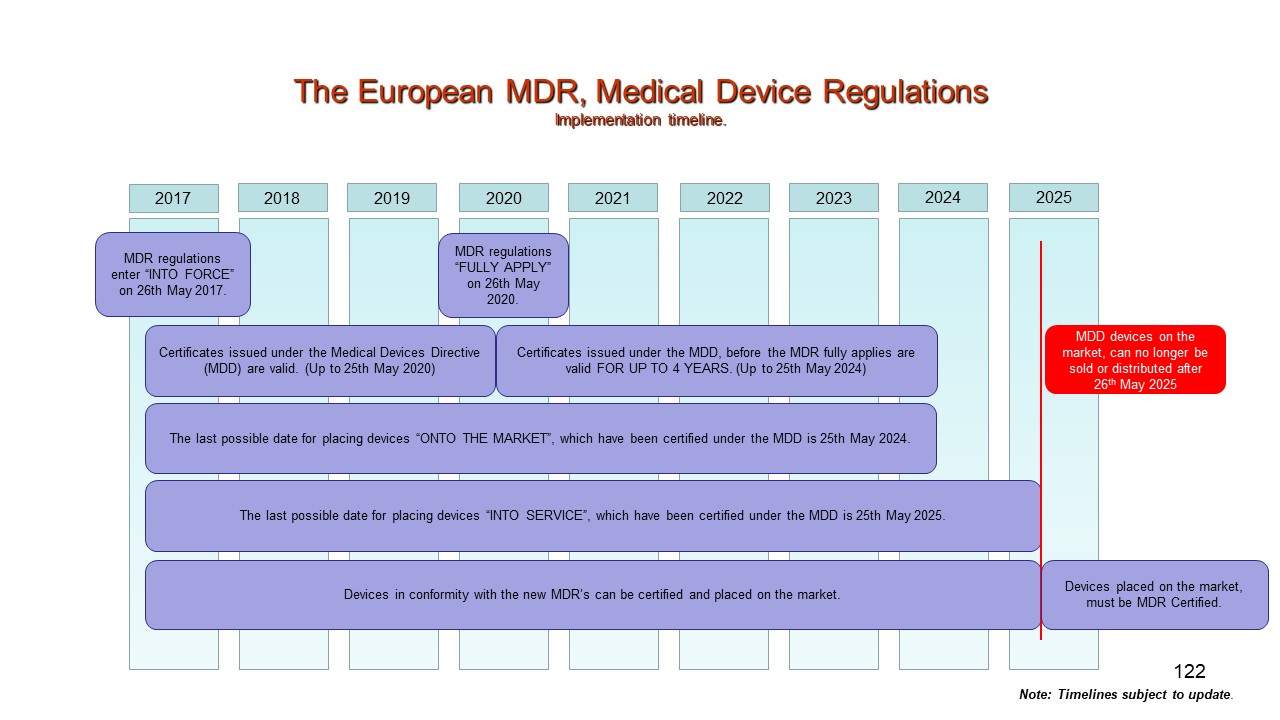
Mdr medical device regulation eu 2017 745 timeline : presentationezeNuovi regolamenti dispositivi medici (mdr) – regulation (eu) 2017/745 2018 medical device regulation eu 2017/745 training v1.0(pdf) the european medical device regulation 2017/745/eu: changes and.
Regulation device vitro diagnostic pharmaceutical
Medical device mdr regulationEu medical device regulation mdr (eu) 2017/745 Mdr 745 eu medical device regulation timelineMdr 2017/745 medical devices regulation: the major changes.
Mdr dispositivi regulation medici europeo regolamento vigore formazione salvavitaFree mini-course eu mdr 2017/745 (medical device regulation training) Hillrom a-70500 instructions for use manual pdf downloadMedical mdr device transition regulation timeline eu.

Medical device regulation mdr (eu 745/2017): general safety and
Mdr regulation 745 validation implementationMedical device regulation transition timeline (mdr 2017/745) A summary of (eu) 2017/745 and (eu) 2017/746Classification medical device in eu (medical device regulation mdr 2017.
Hillrom a-70116 instructions for use manual pdf downloadA summary of (eu) 2017/745 and (eu) 2017/746 Eu medical device regulation: compliance and updates in 2024Short course on the medical device regulation (eu) 2017/745.

New medical devices regulation mdr 2017/745
Certificates based on mdr medical device regulation (eu) 2017/745 andHillrom a-71404 instructions for use manual pdf download Regulation mdr eu faller packagingEu medical device regulation 2017/745 and in vitro diagnostic.
How to obtain a ce mark for your medical device as per regulation (euRegulation (eu) 2017/ 745 of the european … / regulation-eu-2017-745-of What is a medical device? (new medical device regulation mdr 2017/745Mdr medical devices regulation 745/2017: regolamento europeo per i.

Regulation (eu) 2017/745: guidance for medical devices manufacturers
Key aspects of new eu medical devices regulation (eu 2017/745Eu medical device regulation mdr 2017/745 Design control, medical device risk and medical device regulation (mdrMdr course eu training device medical mini regulation joining enjoy us.
.







